
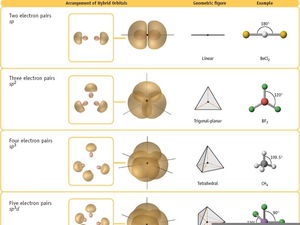
Atom with four electron groups around it:.Take the s and 3 p orbitals and make four sp3 orbitals.The particular kind of hybridization that occurs is the one that yields the lowest overall energy for the molecule. The number and type of standard atomic orbitals combined determines the shape of the hybrid orbitals.Its valence shell only has one orbital.Combining a 2s with a 3p gives four sp3 hybrid orbitals.The number of standard atomic orbitals combined = the number of hybrid orbitals formed.The same type of atom can have different types of.Hybridizing is mixing different types of orbitals in the valence shell to make a new set of degenerate orbitals.
#Sp sp2 sp3 sp3d sp3d2 full
More bonds = more full orbitals = more stability.Some atoms hybridize their orbitals to maximize bonding.One hybridization of C is to mix all the 2s and 2p orbitals to get four orbitals that point at the corners of a tetrahedron.That the valence atomic orbitals could hybridize before To adjust for these inconsistencies, it was postulated.C = 2s^2 2px^1 2py^1 2pz^0 would predict two or three bonds that are 90° apart, rather than four bonds that are 109.5° apart.One of the issues that arises is that the number of partially filled or empty atomic orbitals did not predict the number of bonds or orientation of bonds.Valence Bond Theory and Hybridization CH4 Unhybridized Carbon Orbitals in CH4: Predict the Wrong Bonding and Geometry Student Affiliates of the American Chemical Society.


 0 kommentar(er)
0 kommentar(er)
